AI-Powered
Site Monitoring
for Faster, Safer Trials
Replace reactive monitoring with AI-powered protocol oversight that detects risks in real-time, automates CRA workflows, and transforms trial chaos into predictable performance
Smarter Central Monitoring: A New Standard
Espresso introduces a smarter monitoring experience that helps improve site performance and data quality from day one.

Catch issues while they’re still small.
Our AI monitors site inputs, visit logs, and documents to flag high-risk protocol deviations and missing actions - before you even expect them.

Support your CRAs without adding admin.
Espresso auto-generates visit prep checklists, assigns follow-ups, and audit-ready summaries
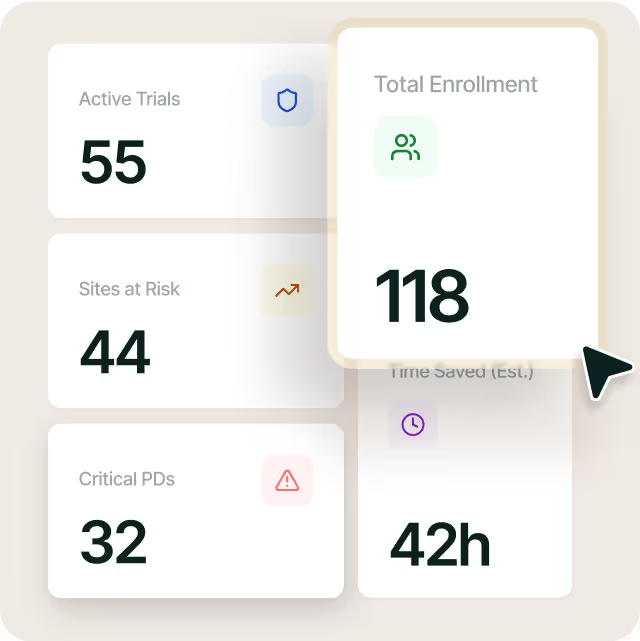
Know what matters most in one quick view
Visualize trends on site performance, deviation scoring, and unresolved actions. No toggling between dashboards

Stay aligned with GCP and ICH E6(R3).
Track decisions, actions, and site history with full visibility. Everything is tagged, time-stamped, and inspection-ready.

Connect via API, deploy in days, not weeks.
No new portals, no new passwords, no IT delays.
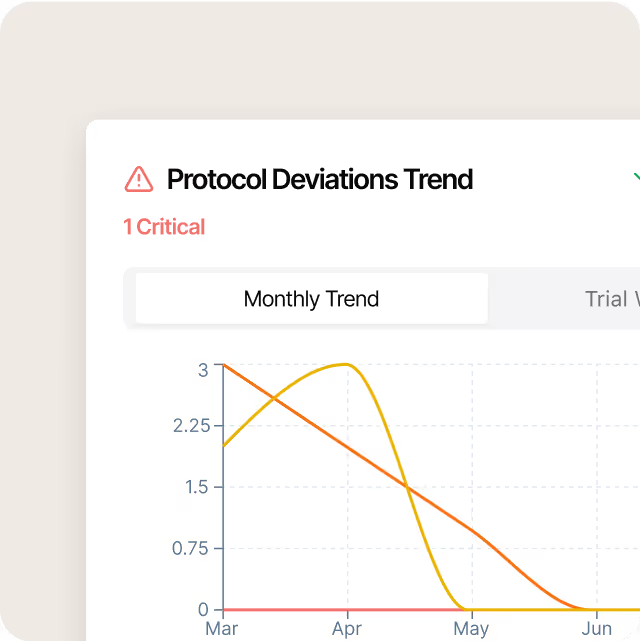
Don't stop at detection
Assigns owners, tracks completion, and reminds teams, so follow-ups get done faster, and deviation loops actually close.
From Passive Oversight
To Active Control
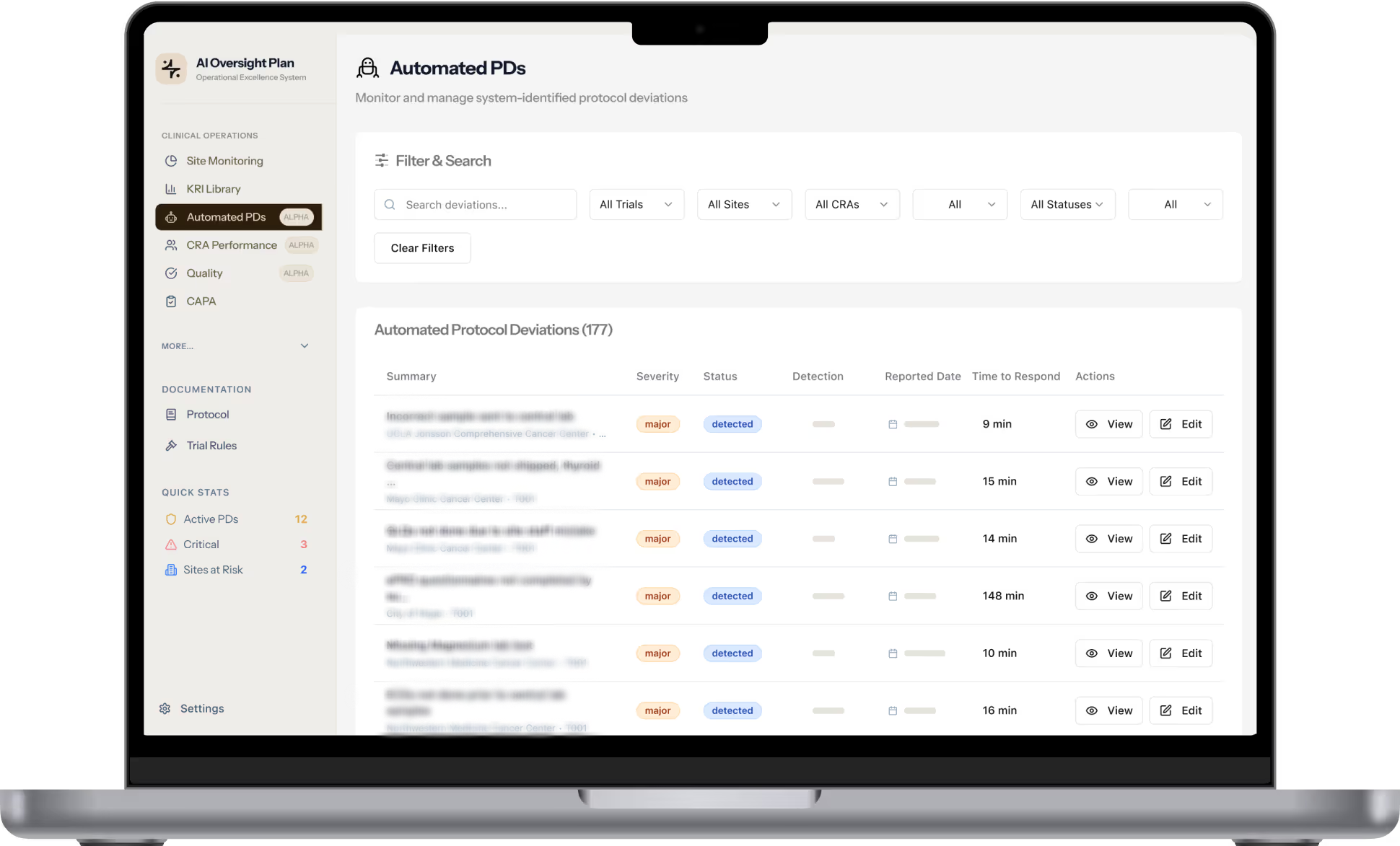
Empower Clinical Ops with Real-Time Execution
Espresso goes beyond dashboards - it actively augments your clinical workflows with intelligent AI agents. By shifting from static site reports to dynamic, data-driven oversight, your team gains faster visibility, smarter alerts, and more control over protocol compliance and task completion.
Ready to Modernize Your Clinical Operations?
See how Espresso Clinical can help your team reduce risk, save time, and elevate site monitoring quality - with the power of AI.

What Espresso Can Do for You?
Turn operational complexity into speed, accuracy, and scale 🫰with one AI-powered platform.
Let AI agents take on manual follow-ups, discrepancy checks, and protocol tracking — so your team can focus on decisions, not documentation.
Spot emerging issues in real time with AI-driven insights — resolve problems before they escalate.
Unify processes across all sites — ensure consistent monitoring, reporting, and audit readiness.
Automate reminders, track responses, and confirm resolution — no missed actions, ever.
Get a 360° snapshot of every site’s performance, compliance status, and operational health.
Leverage predictive models to surface hidden risks early — keep your trials on track and inspection-ready.
AI Agents, Built for Clinical Ops
Espresso plugs into your existing trial documents—no integrations, no new portals, no extra lift.
Connect
1. Connect Your Data
Upload reports, logs, and site notes. Espresso starts working right away.
Deploy
2. Deploy Intelligent Agents
AI begins tracking activity, surfacing risks, and suggesting follow-ups.
Monitor
3. Monitor in Real Time
You’ll see protocol issues and CRA actions as they happen—not days later.
SCALE
4. Scale and Standardize
Run more sites with less friction. Espresso keeps every trial aligned and audit-ready.
IMPROVE
5. Repeat with Confidence
Each trial makes the next one smarter. Build momentum across studies without resetting the wheel.
Ready to Modernize
Your Clinical Operations?
See how Espresso Clinical can help your team reduce risk, save time, and elevate site monitoring quality — with the power of AI.

Frequently Asked Questions
Espresso is an AI-powered platform that helps clinical teams monitor trial sites, detect risks early, and streamline CRA workflows — all in real time.
Our AI agents act like digital assistants. They analyze site data, flag potential issues, and prepare follow-ups — helping your team save time and reduce errors.
Not at all. Espresso enhances your team’s work by automating the repetitive tasks and providing insights — so they can focus on higher-value decisions.
We work with standard trial documents like monitoring visit reports, deviation logs, and site communications. No need to change your existing systems.
Yes — our platform is built with GCP and FDA expectations in mind, focusing on quality-led processes and audit-ready documentation.
Most teams can be up and running in just a few days. We offer flexible onboarding with minimal lift for your ops or data teams.
Still have questions?
We’re only a message away, Contact us anytime.
